This app is intended for use by U.S. healthcare professionals. You will need a redemption code from your AbbVie sales representative to launch the app on your iPad.
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Please see full Prescribing Information for LUPRON DEPOT® (leuprolide acetate for depot suspension) at http://endofacts.com/prescribing-information.cfm
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Endofacts: Road to Relief is an educational tool designed for healthcare professionals to explain endometriosis and therapy options to their patients.
This engaging, interactive iPad presentation visually “walks” patients through the information and addresses their concerns in a friendly, non‐technical way.
Use the app during consultations with your patients to help them understand what they can expect. As you discuss different aspects of endometriosis diagnosis and treatment, an animated female figure walks along a timeline representing the patient’s journey.
The app includes these sections:
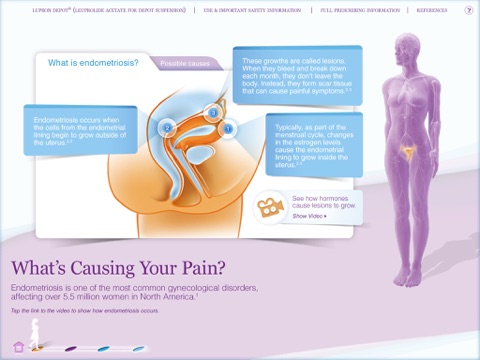
• What’s Causing Your Pain? Use this section to explain the causes of endometriosis. Show patients how hormones cause the growth of lesions with an easy‐to‐follow video.
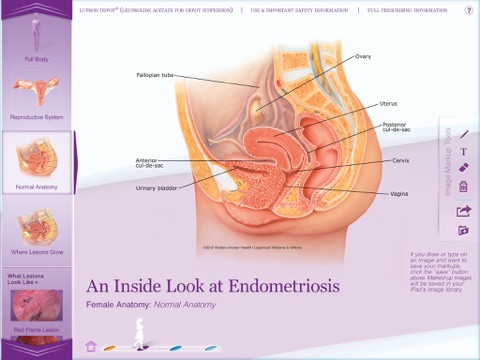
• An Inside Look at Endometriosis. Select from a library of medical illustrations and actual lesion photography, or upload your own images, to explain the different types of lesions; show your patients where lesions can be found in the body; and explain why surgery does not always remove all of the lesions. Use an iPad stylus or your finger to draw or add notes on the images and point out what’s important. You can also email images to patients.
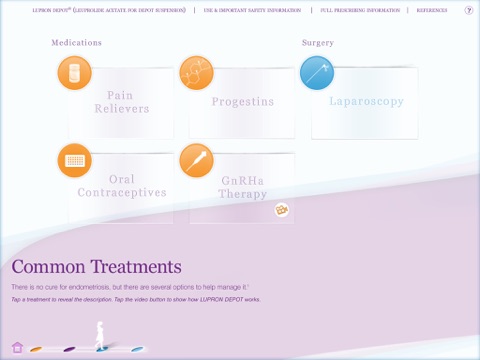
• Common Treatments. Explain the medications and surgical procedures that are most commonly used for endometriosis relief, including LUPRON DEPOT. A short video shows patients how LUPRON DEPOT works and what they can expect during the initial and continuing weeks of treatment.
• Side Effects & Add‐back (norethindrone acetate 5 mg) Therapy. This section allows you to clearly explain the side effects of treatment with LUPRON DEPOT and how Add‐back* can help. You can show patients how certain side effects are reversible at the end of LUPRON DEPOT treatment, and address patients’ fears about menopause.
This app is not intended to be a substitution for your independent professional judgement. Click on the link below to access Indication, Important Safety Information and Full Prescribing Information.
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
Indication for LUPRON DEPOT
LUPRON DEPOT® (leuprolide acetate for depot suspension) 3.75 mg and –3 Month 11.25 mg are indicated for the management of endometriosis, including pain relief and reduction of endometriotic lesions. LUPRON DEPOT with daily norethindrone acetate 5 mg is also indicated for initial management of endometriosis and for management of recurrence of symptoms. Duration of initial treatment or retreatment should be limited to 6 months.
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
General Safety Information for LUPRON DEPOT
LUPRON DEPOT is contraindicated in women who have undiagnosed vaginal bleeding, who are or may become pregnant or are breast‐feeding. Nonhormonal methods of contraception should be used. An increase in clinical signs and symptoms may develop during the initial days of therapy. Development or worsening of depression has occurred. Bone loss over the course of treatment can occur and may not be fully reversible. Prescribers need to be familiar with the multiple contraindications and serious risks associated with the use of norethindrone acetate as add‐back therapy with LUPRON DEPOT. See the Important Safety Information link below for add‐back risks and considerations.
*Norethindrone acetate 5 mg daily